Cell electrode inert electrochemical anode voltaic example chemistry conventions cathode libretexts fe active cells cu Voltammetry polarography electroanalysis electroanalytical potentiometry inert electrodes Igcse chemistry 2017: 1.58c: describe experiments to investigate
4 Inert Electrodes - YouTube
Electrolysis inert electrode chemistry
Ch. 20-3 inert electrodes/voltaic cells
Electrode active example explain help copper inert difference used betweenWhat is electrolysis in chemistry-animation Electrolysis inert electrodes affectingElectrode inert electroanalytical methods ppt powerpoint presentation.
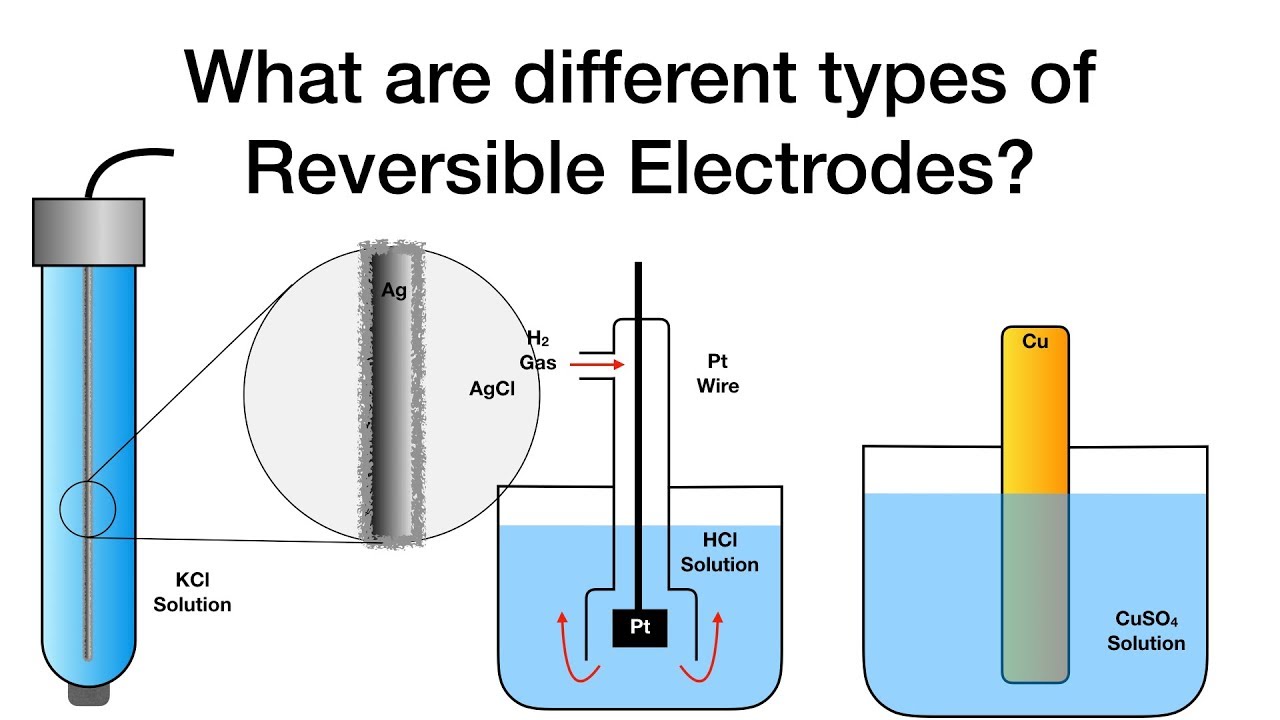
Standard electrode potentialStandard potentials 6.2: standard electrode potentialsElectrodes chemistry reversible electrochemistry types different physical.

4 inert electrodes
Inert electrolyte electrodesChemistry galvanic cells cell voltaic platinum inert beaker wire general magnesium electrochemical diagram mg cu zn pt half electrodes solution Electroanalytical chemistry potentiometry voltammetry and polarographyElectrolysis bromide lead igcse ii aqueous solutions chemistry experiments diagram copper molten electrode inert using chloride acid sulfuric investigate dilute.
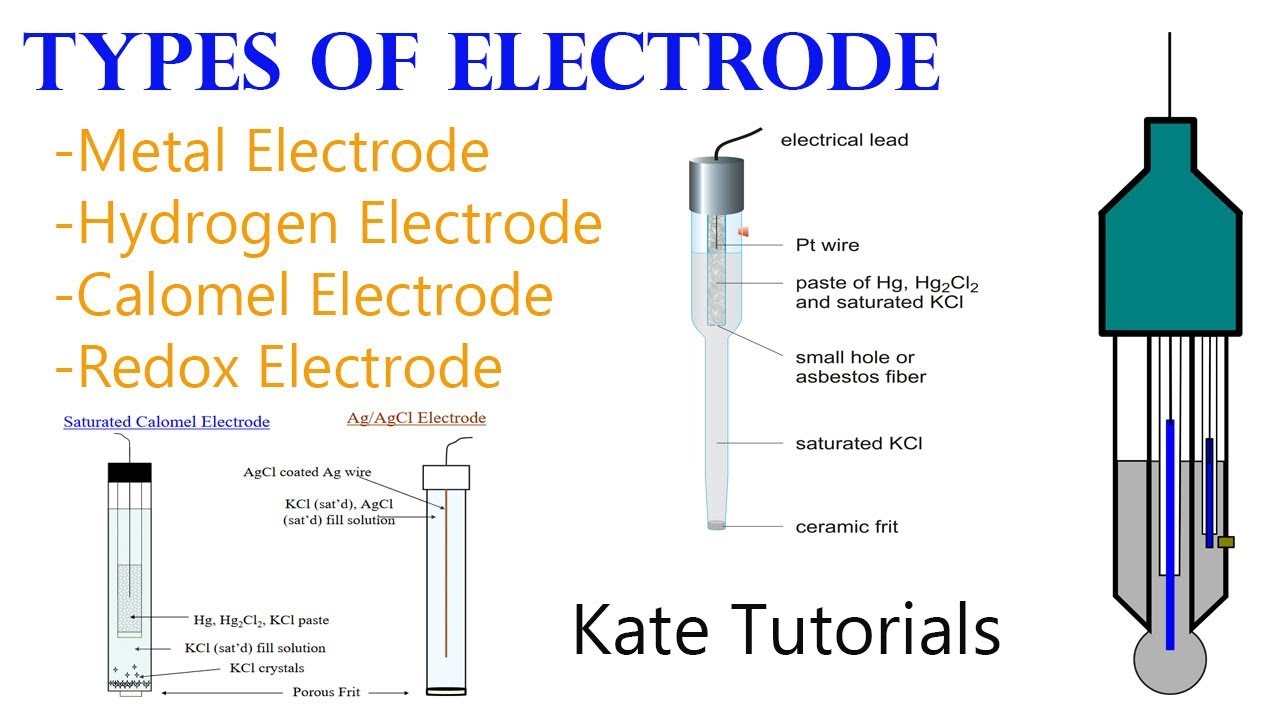
Standard potentials potential electrode hydrogen using chemistry cell measuring determining6 different types of electrodes & their reactions in electrochemistry Electrochemical cell conventionsCopper electrolysis using molten sulphate electrodes inert explain dear student reactions equation occuring tell both also.

Electrolysis electrodes inert reactive cuso4
Standard potential reduction electrochemical potentials chemistry cell half figure galvanic cu she cu2 used pb using electrode hydrogen diagram alElectrolysis electrodes inert ions appropriate attract Factors affecting electrolysisElectrolysis inert electrodes solution using aqueous solutions gif apparatus tubes originally filled small chemguide.
Electrode inert electrodes typesInert electrodes electrolysis active difference between copper chloride natural diagram electrode graphite example vs setup grade atoms decompose sciences figure Electrolysis sulfate electrodes graphite equationsWhat is an active electrode. explain with the help of an example..

Potential electrode standard hydrogen measured byjus definition chemistry
Inert electrodes voltaic cellsElectrolysis cell electrolytic chemistry reactions electrochemistry adichemistry material used examples Electrolytic cellsElectroylsis battery, electrodes and electrolyte (inert electrodes.
Electrodes types different electrochemistry their reactionsExplain electrolysis of molten copper sulphate using inert electrodes Electrolysis of solutions with inert electrodesInert electrochemistry ppt powerpoint presentation anode.

Electrolysis molten chloride sodium cell electrolytic compounds diagram ion battery cells electrode ionic ions negative cathode electrochemical compound na electricity
Standard cell potentials electrode hydrogen chemistry potential electrochemical electrochemistry half reaction pt metal she reduction science cathode platinum surface conditionsClass xi-xii : electrolysis of aq. cuso4 using inert electrode Standard reduction potentialsCuso4 electrolysis using electrode aq inert.
Difference between active and inert electrodesWhat are different types of reversible electrodes? .